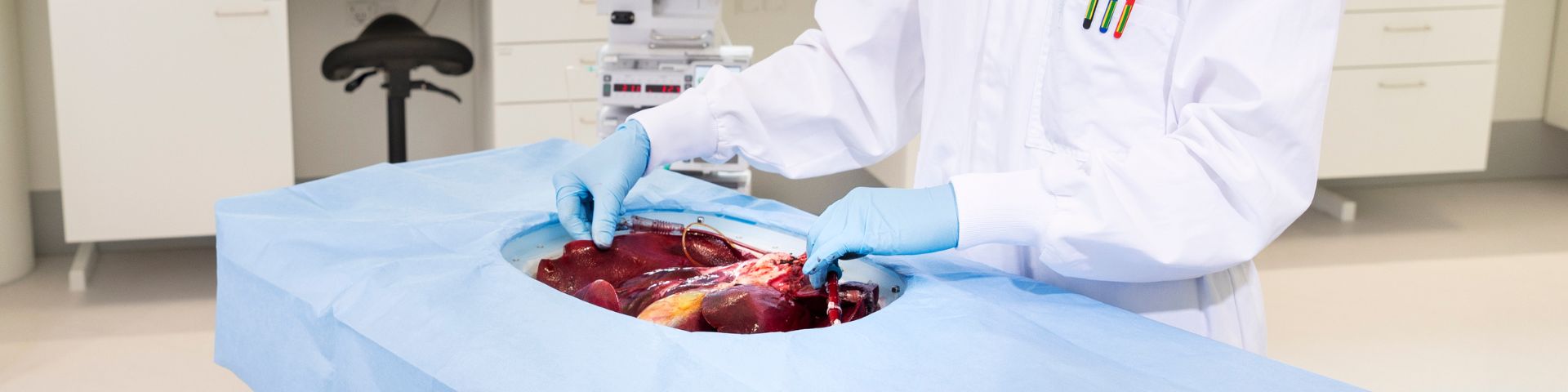
Testing medicines outside the body: intestinal–liver–kidney model accelerates drug development
Wouldn’t it be ideal to observe how a medicine behaves in organs like the liver, intestines, and kidneys before testing it in living humans or animals? TNO’s new multi-organ perfusion model makes this possible. The model keeps multiple organs alive outside the body using organ perfusion, connected just like in a real human body. This research recently won the Best Paper of the Year Award 2024 from the European Journal of Pharmaceutical Sciences.
For the first time: the complete drug pathway
In this study, TNO, in collaboration with transplant surgeons from LUMC, combined the intestine, liver, kidney, pancreas, and spleen in a single perfusion system for the first time. ‘Usually, we only perfuse the liver or kidney,’ explains Evita van de Steeg, senior scientist at TNO. ‘But here, we perfused several organs together, as they are hormonally interlinked.’
The system closely mimics the natural journey of a drug through the body: administration via the intestine, absorption through the intestinal wall, transport to the liver, and finally distribution through the bloodstream and excretion via the kidneys.

‘It’s unique that we managed to connect all these organs to a single perfusion pump. A surgeon was present to make it happen.’
Organs in natural balance
A major advantage of the combined model is that the organs maintain each other’s homeostasis. ‘If you only use a liver or kidney, you often need to add extra glucose,’ Van de Steeg explains. ‘But with the pancreas included, there’s better hormonal balance, and we see that glucose levels and acid–base balance are much better regulated, resulting in a more natural equilibrium.’ This natural homeostasis between organs creates a more realistic testing environment than isolated organs can provide.
More accurate predictions, faster development
Developing a new medicine typically takes 10 to 15 years. TNO aims to shorten this process by two years. Organ perfusion contributes to this goal. ‘This ex vivo platform can predict eventual drug-drug interactions, accelerating and de-risking further clinical development,’ emphasises Steven Erpelinck, principal business developer at TNO.

‘We study processes in the entire organ, and the data obtained are predictive of what happens in humans.’
These detailed insights enable pharmaceutical companies to make faster and better-informed decisions. Moreover, the model helps reduce animal testing, as it uses slaughterhouse waste (pig organs) or human organs rejected for transplantation.
Also for gene therapy, radiotoxicity, and metabolite production
The model is not only useful for studying the pharmacokinetics and metabolism (breakdown) of drugs (also known as DMPK). It can also be used for gene therapy, where genetic material such as RNA is introduced into a patient’s cells to replace, repair, or compensate for defective genes. Erpelinck: ‘We then check whether the correct gene reaches the right place in the cell and whether it is properly integrated so the cell or organ can function again.’
Erpelinck adds: ‘We can also use our organ platform for targeted radiotherapy against cancer. Certain radiopharmaceuticals tend to accumulate in the kidney. With organ perfusion, we can study potential kidney toxicity by perfusing the organ for a specific period.’
Finally, there is another unique application of the multi-organ perfusion model. Van de Steeg: ‘In that application, we essentially use the model as a factory for metabolite production.’ These metabolites can then be used for other purposes. ‘I also call it a ‘metabolite production platform’, and we’ve now applied for a patent on it.’
Extending organ viability outside the body
Extending the lifespan of perfused organs is a major challenge. The longer organs remain viable, the longer research can be conducted. This is also literally vital for organ transplantation. That’s another goal TNO is working on—with promising results.

‘We’ve already managed to keep a liver alive for two days. Normally, that’s about 8 to 10 hours. We only had to stop because we ran out of resources—the liver itself was still functioning well.’
Further expansion of the organ model
There are more ambitious plans for the future. ‘We want to expand the perfusion model with other organs. The intestine is particularly interesting. A section of the intestine was already included in the model, but there are exciting challenges in expanding this to multiple intestinal regions (both small and large intestine) so that digestion and regional processes in the gut can be studied. This is a crucial process for drug absorption, and there are currently few, if any, suitable models for it,’ says Van de Steeg. ‘The placenta also offers opportunities. The FDA is very interested in knowing which drugs can cross the placenta.’
Interested in collaborating?
As the only independent European research and technology organisation, TNO makes its preclinical tissue models available to Pharmaceutical and Biotech companies. The multi-organ model is particularly valuable for pharmaceutical companies that want to understand how their drug and potential metabolites behaves in the body at an early stage and thus accelerate their time-to-market. It can also be used during later-stage clinical studies to investigate and understand unexpected results.
TNO has already established successful collaborations in organ perfusion with partners such as LUMC and Takeda and is eager to address other unmet needs within the R&D projects of companies. TNO is open to new ideas. Erpelinck: ‘Ultimately we want to best mimic pharmacokinetic profiles of new compounds in the human body thereby accelerating the drug development process.’
Get inspired
Preclinical ADME


TNO launches Peregrion to boost market impact of its technology that accelerates medicine development


PPP uncovers new insights into MASLD development


Translational preclinical efficacy models


Time setters: reduce long waits for new medication with AMS



