
ML-III Facility for research on pathogenic microorganisms
The Microbiology and Systems Biology (MSB) department, part of the TNO unit Health & Work, boasts a state-of-the-art ML-III facility. This facility enables safe and controlled research on pathogenic microorganisms.
The ML-III facility comprises two separate laboratories: one for viral research and one for bacterial and fungal research. In these laboratories, we study class 3 (BSL-3) microorganisms and GMOs at containment level ML-III. This includes research on coronavirus (SARS-CoV-2), tuberculosis (Mycobacterium tuberculosis), Shiga toxin-producing E. coli (STEC), and blastomycosis (Blastomyces dermatitidis). The facility supports applied research on infectious diseases, antimicrobial resistance, the development of antiviral therapies, and testing the effectiveness of disinfectants.
Unique infrastructure
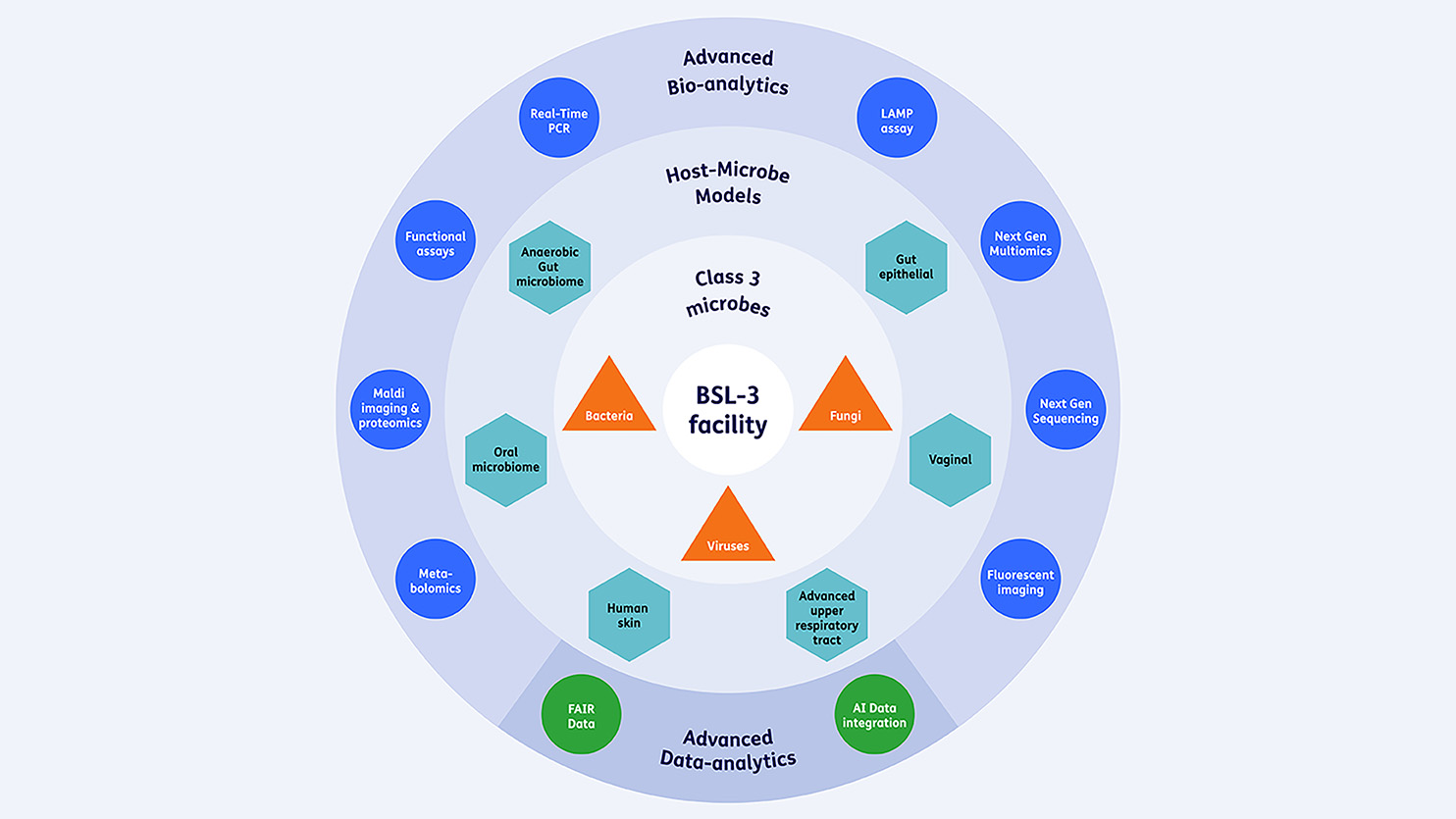
The facility at TNO in Leiden is unique due to the integration of research on class 3 microorganisms, application of host-microbe in vitro models, advanced bioanalyses, and data analyses (Figure 1). To achieve this, TNO is equipped with innovative analytical equipment that can be utilized in research projects.
Work safety
The facility meets all safety requirements according to current regulations, is 100% airtight, and has separate ventilation and filtration systems, isolated from the rest of the building. Everything leaving the lab is autoclaved, and water is separately collected and disinfected. To further comply with regulations and ensure a safe working environment, access is highly restricted, extensive training is provided, staff wear protective clothing, and work is always conducted with at least two people.
Research examples in the ML-III facility
Gut microbiome research
Commissioned by the Ministry of Health, Welfare and Sport (VWS), we are conducting research focused on the role of the microbiome in post-COVID patients. This project examines the gut microbiome composition of people with post-COVID and evaluates the effectiveness of microbiome-modulating interventions in post-COVID (host) microbiome in vitro models.
Shiga toxin-producing E. coli (STEC) research
In this project, we have isolated E. coli bacteriophages that can infect and kill STEC strains. People can become infected with Shiga toxin-producing E. coli (STEC) bacteria through food (such as eating undercooked beef) or contaminated manure. Many animals, such as cattle, sheep, pigs, and poultry, have STEC in their intestines. Bacteriophages have high specificity, enabling targeted antibacterial therapy. This makes bacteriophages a promising alternative for treating bacterial infections.
Want to learn more?
Would you like more information about co-innovation opportunities or have a research question? Please contact us.
Get inspired
Testing medicines outside the body: intestinal–liver–kidney model accelerates drug development


Preclinical ADME


TNO launches Peregrion to boost market impact of its technology that accelerates medicine development


PPP uncovers new insights into MASLD development


Translational preclinical efficacy models




