Animal testing policy
Safe and healthy food, medicine, consumer products, and household chemicals, both at home and at work, are important to all of us. Research at TNO contributes to the development of these products and helps us predict whether substances are safe and effective. Sometimes animal testing is a necessary part of this research, partly due to statutory requirements and partly because there are not yet any alternatives.
Table of Contents
Click on the topic to jump directly to that section on the page.
Our animal testing policy
Transparency and communication
Our ambition
Facts and figures
Our animal testing policy
We have formulated a meticulous animal testing policy, based on the following principles:
- we only carry out animal testing if required by law or if no reliable alternatives are available;
- we contribute actively to the development and application of alternatives to animal testing (the three Rs: Reduction, Refinement, Replacement);
- we adhere strictly, of course, to the statutory rules and regulations.
Intrinsic value of animals
We attach great importance to the intrinsic value of each individual animal. That is why all experiments are subject to ethical review and careful treatment of the laboratory animals is paramount.
Animal testing is only permissible when there is no suitable alternative and the purpose of the research outweighs any distress for the animal. A decision on this is taken for each individual study, weighing up the ethical necessity. Animal welfare is ensured subject to strict legislation by an independent Animal Experiments Committee (AEC).
In addition, we actively participate in the social debate on the use of laboratory animals. We are well aware of developments in the field of animal testing that contribute to fewer animals or better living conditions and are keen to apply them ourselves.
Our policy
Our policy on the use of laboratory animals is part of our TNO Corporate Social Responsibility policy and is the official guideline for the TNO management and employees. The Animal Welfare Body (AWB) evaluates the policy annually and reports directly to TNO's Executive Board.
Animal welfare
Laboratory animals are entitled to optimal care throughout their lives. We agree with the view that certain circumstances justify the use of laboratory animals. We also realise that by carrying out animal experiments, the researcher and TNO’s Executive Board are responsible for the ethical considerations and animal welfare. TNO's Animal Welfare Body and our employees together ensure a 'culture of care' for laboratory animals. All animals are monitored daily, and there are special procedures if an animal requires additional attention.
Reduction, replacement, and refinement
Animal testing is only permissible when there is no suitable alternative and the purpose of the research outweighs the animals’ potential distress. The main principles are the reduction, refinement, and replacement of animal testing (3R). We operate within the boundaries of relevant legislation and, where applicable, according to quality criteria such as those of the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC).
Continuous improvement of methods
Animal testing has contributed to significant advances in science, health, and well-being. Despite the results achieved, there is a continuing need for methods with better-substantiated predictive value for humans. Further development and implementation of innovative 3R methods offers opportunities for improving existing procedures. This approach is firmly embedded in our policies, research programmes, and communications.
Policy document
TNO’s policy document (pdf, 2.1 MB) on animal testing sets out TNO’s policy on the use of laboratory animals in research. It emphasises careful decision-making, legislative compliance, and ethical considerations, based on the principle that non-animal alternatives are always preferable. TNO strives for transparency, continuous improvement of animal testing, and active involvement in the development of new alternative methods. The policy applies to all TNO employees and includes guidelines for applying for permits, supervision by the Animal Welfare Body, and communication with stakeholders.
Transparency and communication
For us, transparency and communication are essential. We provide clear information about our activities and our position on animal testing in order to establish a dialogue based on mutual respect.
This dialogue is strengthened by the objective exchange of information (without compromising the safety of researchers and laboratory animals or the confidentiality of research data), and maximum transparency.
Central to this Transparency Agreement is that TNO engages with the various stakeholders on all aspects of animal testing. Openness about laboratory animal use and animal testing is one of the key issues in the corporate social responsibility (CSR) policy.
Transparancy
TNO is transparent about the use of animal testing, and we consequently endorse the Transparency Agreement on Animal Testing in the Netherlands. Following on from the ‘Open-by-Design’ pilot, to which TNO also contributed, we have decided to publish all approved applications for animal testing (from 2016 onwards) on this website.
Applications approved by the Central Authority for Scientific Procedures on Animals (CCD)
Medical countermeasures, toxicology and safety.
- Niet technische samenvatting (pdf)
- Aanvraagformulier (pdf)
- Projectvoorstel (pdf)
- Omschrijving dierproeven 1 (pdf)
- Omschrijving dierproeven 2 (pdf)
- Omschrijving dierproeven 3 (pdf)
- Omschrijving dierproeven 4 (pdf)
- DEC-advies (pdf)
- Beschikking en vergunning (pdf)
- Melding 1 (pdf)
- Melding 2 (pdf)
- Toelichting melding 2 (pdf)
- Beschikking naar aanleiding van melding 2 (pdf)
- Verlenging project (pdf)
Studying the function and pharmacokinetics in organs (intestine/liver/kidney) for scientific research.
Metabolic diseases, animal model, drug development, metabolism.
Determining and validating the effectiveness of new drugs and investigating the mechanisms involved in inflammation and fibrosis processes in animal models.
- Niet-technische samenvatting (pdf)
- Aanvraagformulier (pdf)
- Projectvoorstel (pdf)
- Beschrijving dierproeven 1 (pdf)
- Beschrijving dierproeven 2 (pdf)
- Beschrijving dierproeven 3 (pdf)
- Beschrijving dierproeven 4 (pdf)
- DEC-advies (pdf)
- Beschikking en vergunning (pdf)
- CRO voorwaarde (pdf)
- Reactievoorwaarde (pdf)
- DEC-voorwaarde (pdf)
Ensuring the quality of animal experimental research.
Research for the development of medical products that affect the immune system.
The knowledge about the origins, prevention and treatment of metabolic diseases early in life and their consequences later in life.
Testing medicines and other substances for cardiovascular-related side effects.
Threat of highly toxic substances, effectiveness and side effects of medical countermeasures.
Studying the effects of substances in pig intestine, liver and/or kidneys.
Ensuring the quality of animal experimental research.
Research into new medicines against scarring and inflammation using mouse models.
The prevention and treatment of metabolic diseases.
The prevention and treatment of muscle loss.
Publications
We publish transparently and systematically about our animal testing, as laid down in the ARRIVE guideline (Animal Research: Reporting of In Vivo Experiments).
Our staff have also endorsed the Montreal Declaration on the Synthesis of Evidence to Advance the 3Rs Principles in Science, and we comply with the Netherlands Code of Conduct for Research Integrity (NGWI). In addition, our animal testing-based publications contain the information essential according to international guidelines in order to prevent the repetition of animal testing and to enable meta-analysis.
Our ambition
Our ambition is to conduct excellent biomedical research aimed at improving human health. Both clinical and pre-clinical technologies are developed and applied for this purpose. In all our pre-clinical methods – including in silico and in vitro methods as well as animal testing – the emphasis is on the predictive value for humans.
New 3R methods
It is our ambition to develop new innovative 3R methods and to have these methods accepted for application more quickly. We therefore engage and collaborate with all relevant parties including academics, legislators and regulators, public authorities, and industry. We deploy our own research tools to replace, reduce, and certainly refine animal testing.
Accelerating the transition to non-animal research
We wish to accelerate the transition to non-animal research and are working on new technologies to do so. Once we have found ways to improve research methods, it becomes our top priority to gain wide acceptance and actually implement these 3R improvements.
‘Organ-on-a-chip': human stem cells are cultured on a chip to mimic specific human organ functions.
‘Microtracing’: (also known as 'microdosing') this is a highly specialised technique involving the administration of very small, non-harmful quantities of medication to humans. It can help study what the human body does with the medication and vice versa. This greatly reduces the need for animal testing prior to the clinical phase.
‘In silico’ systems, i.e. computer-based simulations and models that mimic biological processes. These systems enable predictions about safety and effectiveness in advance, thus reducing the need for laboratory testing.
Research using ex-vivo human material. We are a partner in the VitalTissue project. This involves utilising remnant human tissue as valuable research material.
Innovations
The Netherlands aims to be at the forefront of the international transition to non-animal innovation. We work together with companies, public authorities, academic institutions, and civil society organisations on this transition by developing and applying technologies that contribute to achieving that goal. For example, we are working with our partners on the Transition to Non-Animal Innovation (dutch: TPI) project initiated by the Dutch government, with the aim of speeding it up.
Our role at TNO is to look ‘beyond the hype’, by tackling the scientific foundations of new research and matching the supply of innovations with industrial demand through contacts with industry so as to achieve practical implementation.
Replacement, reduction, and refinement of animal testing (3R)
Reduction
We aim to reduce the number of animals used for testing. We regularly evaluate our testing methods and implement integrated testing strategies. This is how we determine whether animal testing is necessary or whether the same information can be obtained by other means. In addition, for experiments in which only tissue from laboratory animals is needed (but not the intact animal), animals from other experiments or from control groups (within or outside TNO) are used as far as possible, so that laboratory animals are used as optimally as possible.
Refinement
We strive to develop and adapt testing methods to minimise discomfort and stress to laboratory animals. We do this by using state-of-the-art techniques and continuously optimising suitable human and experimental endpoints.
Replacement
We aim to replace animal testing with other methods, preferably using human tissue and cells. Wherever possible, we use human samples. Examples of this are:
TIM: TNO’s unique gastrointestinal models for research on digestion of food or drug kinetics. We are trying to use this to replace animal testing with alternative research methods.
VitalTissue: the use of remnant tissue from the clinic to perform laboratory tests.
Encouraging employees
At TNO, we foster a stimulating environment in which our employees are encouraged to contribute their own ideas to make research more animal friendly. If a method offers improvement while maintaining the same research results, we make the new method available to others.
Award-winning
Encouraging employees to contribute ideas is also paying off. In 2021, we were given an award for refinement of animal testing at the DALAS symposium. This was before all optimisations made by the animal testing researchers to the diabetic renal failure model. These optimisations included the introduction of ‘tube-handling’ which eliminates lifting the animals by the tail, heated racks, and improved measuring methods. All these aspects enabled the animals’ distress in these tests to be significantly reduced.
Our collaborative partnerships
In order for parties involved in legislation and regulation to think differently about animal testing and alternatives, it is essential to consider a broader perspective. The whole area needs to change its mindset. An organisation can be a catalyst for change. That is why we are participating in various groups to encourage dialogue and find ways to make the 3R principles work.
Intensive interaction between industry, academia, public authorities, and regulatory authorities is needed to accelerate the acceptance and validation of alternatives. We proactively provide information through scientific publications and lectures. The following are some examples from the network within which TNO or its employees are active.
- Transition to Non-Animal Innovation (TPI).
- Netherlands Animal Testing Information Foundation (SID).
- Netherlands Association of Animal Experiments Committees (NVDEC).
Discussion groups with other interested parties, including animal welfare organisations and political parties, with the aim of sharing our views on animal testing, creating respect, and exploring common goals.
Facts and figures
Animal testing is carried out with the utmost care. Over the past 35 years, the use of laboratory animals in the Netherlands has been more than halved. The number of animals used for testing has been significantly reduced at TNO. After a sharp decline in 2013, thanks to a revised breeding policy, our number of laboratory animals has remained stable in recent years. Although a decrease in the number of laboratory animals was seen in 2020 as a result of Covid-19, this has since continued.
Summary of total number of TNO animal tests per year from 2015.
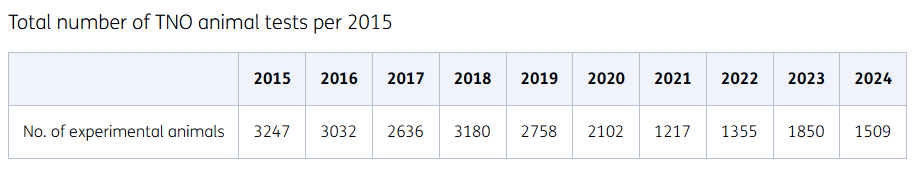
Focus on reducing breeding surplus
We breed the special mice ourselves, making sure that the animals that are born are used as efficiently as possible. One of the ways in which we do this is by coordinating breeding centrally and by joining a national network of breeding coordinators in order to match supply and demand as closely as possible. This prevents a breeding surplus as much as possible.
Number of tests using animals
The following tables show the numbers of animals for the past registration year (2024).
Species
The choice of an animal species for use in an animal experiment depends on its predictive value for humans. They mainly include rodents (mice, rats, and guinea pigs) due to the wide availability of historical data and proven translatability of the models used.
However, rodents are not always the best model for humans and other animal species are therefore sometimes used. For example, a small number of pigs are used as animal models. The use of improved translatable models prevents unnecessary animal testing.
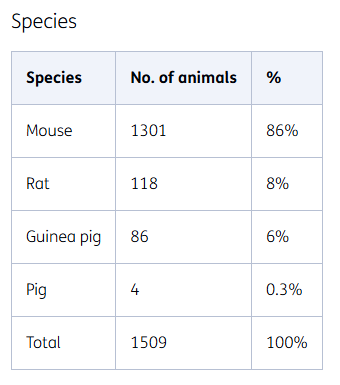
Rodents
In 2023, we mainly used rodents in research. More than three quarters of the mice are transgenic. These animals are implanted with a human gene, so the disease development in these animals is very similar to humans. Much research has been done with these transgenic mice so that we know which aspects of human diseases they are suitable for, and which they are not.
Effective partnerships
If animal organs or tissues are required for research, we do everything we can to avoid killing animals specifically for this purpose. For example, we have a collaboration agreement with several slaughterhouses for the supply of fresh pig tissue. We also have a collaboration agreement with Utrecht University and other institutes in the Netherlands to obtain organs and tissue from animals that have been killed for veterinary training or have been used in studies.
Through these collaborations, we obtained tissue for testing without killing any additional animals. This has been used, for example, for research into the absorption of substances by the intestine, for which, among other things, fresh intestinal material is needed.
In addition, we collaborate with hospitals, which allows us to regularly use fresh human material for research. We are active within the national VitalTissue project, an initiative that helps researchers in the Netherlands to obtain vital remnant human tissue. In addition to the improved translatability of research results, this method contributes to the reduction of animal testing.
Objectives of animal testing
At TNO, we conduct research into societal issues. The results are used to cure and prevent conditions and diseases such as metabolic syndromes, obesity, or cardiovascular diseases. We also study recovery from acute intoxications and the reduction of possible consequential damage. The purposes of animal testing are:
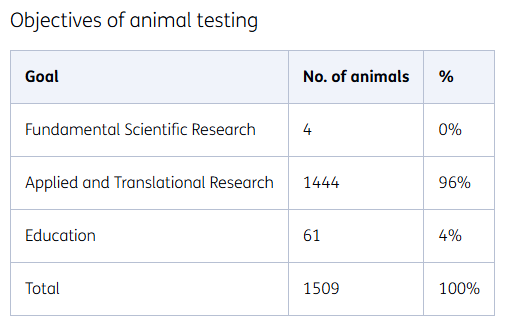
Applied research
The majority of animal tests concern the efficacy (effectiveness) of medicines or therapies. Our applied research is largely into metabolic diseases and protection against intoxication.
Basic scientific research
In addition to applied research, we also conduct basic scientific research into the mechanisms of diseases.
Training of TNO staff
For the education and continuous training of staff, the refinement of research techniques, and the implementation of new research techniques and models, laboratory animals are used on a small scale.
Expertly trained staff contribute directly to the responsible use of laboratory animals. It is important to note that during staff training, animals can usually be used that are not purchased specifically for this purpose, but which were present in the facility and could no longer be used in other experiments.
Animal Welfare Body
An Animal Welfare Body (AWB) has been set up within TNO. This body assesses whether the experiments contribute to the goal defined in the permits. The AWB also supervises the workplace and is a source of information for all animal-related research. The AWB chairperson is not a member of the departments that carry out animal experiments. This establishes an additional independence step. Besides the chairperson, the AWB consists of a designated veterinarian, a laboratory animal pathologist, three researchers, and four laboratory animal technicians.
Prior to animal testing
Before animals are used, an alternative solution is sought. Is the use of animals unavoidable? If so, we look into whether the study can be carried out with fewer animals, or whether methods can be refined. The AWB plays a leading role in this. TNO thus also complies with the frameworks laid down in the national Experiments on Animal Act regarding the internal supervision of animal testing.
Preventing distress
If animal testing is necessary, we strive for the least possible distress for animals and the least possible number of animals required. In most cases, the test animals experience mild or moderate distress. Where possible, we reduce this, for example by earlier humane endpoints, better living conditions (such as cage enrichment), better testing methods, or the use of pain relief.
Distress categories
In a limited number of studies, an animal is anaesthetised for the purpose of the experiment, and waking the animals is not necessary to achieve the results. In this way, the animals experience as little distress as possible. Internationally, such studies are therefore classified separately as 'terminal'.
Other animal experiments are classified as mild, moderate or severe distress according to the general guidelines.
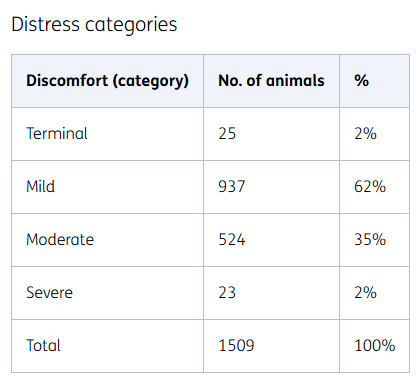
Mild distress
One example of an experiment in the 'mild distress' category is feeding animals a specific diet, for example one that is high or low in fat. The animals are not troubled by this, but they are weighed from time to time, for example.
Moderate distress
In these tests, animals might receive weekly injections of the substance under study, have regular blood samples taken, or undergo a small surgical procedure under anaesthesia. Where possible, procedures are combined to reduce the number of interventions per animal. Pain management is also applied to animals undergoing minor surgery.
Severe distress
This involves testing the efficacy of medication, with the animals having certain symptoms. Inducing these symptoms can lead to ‘severe distress’.
We test medication for such conditions as pulmonary fibrosis and to combat the effects of intoxications. In these studies, we decide on the earliest possible experimental endpoint, so that the experiment can be stopped as soon as acceptable results are obtained, and the animals are in distress for the shortest time possible.
AAALAC accreditation
We consider animal welfare to be of the greatest importance. Therefore, we have AAALAC accreditation for our Leiden location. AAALAC is the Association for Assessment and Accreditation of Laboratory Animal Care, a globally accepted organisation that aims to optimise animal welfare through a voluntary accreditation and assessment programme. This goes beyond the minimum statutory requirements for animal testing.
