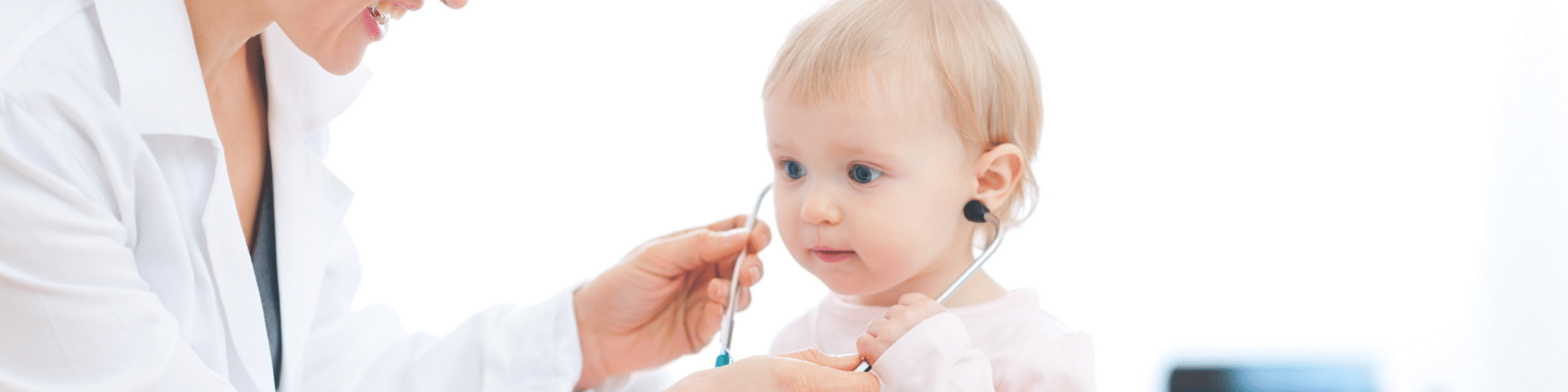
TNO proves method for effective pediatric drug development
Safe and effective drug dosing for young children come with their own challenges. Up to the age of 18, a child’s metabolism can change significantly. And in the first two years of life, those ontogenic differences can be rapid and dramatic. TNO published its findings from the first drug disposition (mass balance/metabolite profiling) study to conclusively prove that microtracing with accelerator mass spectrometry (AMS) is an effective way to collect clinical data in these young patients. This AMS technology can also be the key to building up more data on drug interventions in pregnant and lactating mothers.
Data for reliability
Current pediatric drug development depends entirely on the quality of data that supports them. But generating clinical data for children is far more complex and challenging than for adult patients, from both an ethical and analytical standpoint. Pharmaceutical companies that aim to develop drug protocols for children – especially those under two years old – have faced incredible obstacles and long processes. Defining a safe and effective dose is typically based on a combination of experience, simulations based on physiologically-based pharmacokinetic (PBPK) modelling, and adult dose normalisation based on body weight. But both drug developers and regulators are calling for more accurate means.
‘In terms of metabolic profile, we have actually identified at least three distinct age classes in a child before the age of two years, and total of four before age six,’ explains Esther van Duijn, Senior Researcher at TNO. ‘A compound that may have been toxic to a child at six months can possibly be fine at one year, because, for example, of the developmental changes in metabolism. That’s why thorough study and robust data collection is so important to pediatric drug development.’
Proof of concept
Esther and her colleagues published a Proof of Concept paper that outlined the results of the First Pediatric [14C] Microtracer Study to Create Metabolite Profiles of Midazolam. ‘We chose Midazolam because we already have a significant amount of data about it. But so far, a radio-labelled study with Midazolam in pediatrics patients had never been done, so we never knew the precise route it took in each age group,’ she says. ‘We know it now.’
Receive the proof of concept
First Pediatric [14C]microtracer Study to Create Metabolite Profiles of Midazolam
The Proof of Concept study, published in Clinical Pharmacology and Therapeutics 2021, generated real human adsorption, distribution, metabolism and excretion (hADME) data in groups of children aged 0-1 month, 1-6 months, 0.5-2 years and 2-6 years old. ‘With these AMS studies, we could study all of these age groups at the same time, instead of sequentially as is traditionally the case. This significantly accelerates the collection of essential hADME data and the availability of drugs to children of different age groups,’ Esther explains.
The Midazolam study added a single tracer to patients who were already receiving therapeutic doses of Midazolam. Therefore, there was no risk of toxicity and no effect on the therapeutic effects of the drug. ‘TNO can also perform microdosing studies to estimate a therapeutic level. Our Proof of Concept study of paracetamol confirmed that a microtracing study could generate meaningful pharmacokinetic data,’ Esther explains. ‘We can determine the absolute bioavailability and quantify parent drug concentrations. As dose linearity applies in human adults, it is possible to extrapolate the microdosing data and determine a safe therapeutic dose levels for different age groups. Microtracing offers us a complete metabolic profile and insight into how a drug is processed in the body at any age.’
"TNO has proven that AMS and microtracing is a safe and reliable way to collect essential and potentially life-saving data."
The demand for data
This deeper insight and more targeted exploration are becoming the industry standard in adult hADME studies, and is also recognized for its potential in pediatric drug development. ‘Regulatory agencies require pharmaceutical companies to include a Pediatric Investigation Plan (PIP) for any new compound,’ explains Steven Erpelinck, Senior Business Developer at TNO. ‘And in their final neonate guidelines in 2022, the Food & Drug Administration (FDA) recommended AMS to assess the differences in metabolism based on age. Up until now, drug companies have been limited in how effectively they could provide a PIP or a MIST profile for safety packages. But with AMS, that data can be collected swiftly and reduce a company’s time to market for new drugs and therapies.’
Both Steven and Esther see the benefits of AMS and microtracing for drug development for pregnant and lactating mothers, as well. ‘Several major pharmaceutical companies are pursuing studies of whether compounds are excreted in mother’s milk and/or whether they are transferred to a developing fetus,’ Steven says. ‘TNO has proven that AMS and microtracing is a safe and reliable way to collect this essential and potentially life-saving data.’
Interested in uncovering how much time and effort you can save in collecting pediatric drug data? Contact Esther van Duijn or Steven Erpelinck today to schedule a call.
Free quick scan for your organisation?
Interested in uncovering how much time and effort you can save in collecting pediatric drug data? Contact our expert Esther van Duijn to schedule a call.
Aiming for fast accurate preclinical and clinical trials
Pharma companies want it. Regulators want it. Patients certainly want it: faster, more accurate, less expensive drug development. But with all the complexity that drug development entails, how can we accelerate the process?
About TNO
Innovation for Life
We are TNO. A safer, healthier, and more sustainable life. That's what we are all about. As an independent research organisation, we are the driving force behind innovation. We make knowledge serve the common good. Since 1932, it has been our mission to give the right answers – and to ask the right questions. For the world of today and tomorrow. By combining disciplines and domains, we can tackle the most complex questions. On the road to a better life and a brighter future.
Get inspired
Preclinical ADME


TNO launches Peregrion to boost market impact of its technology that accelerates medicine development


PPP uncovers new insights into MASLD development


Translational preclinical efficacy models


Time setters: reduce long waits for new medication with AMS



