TNO is developing a fast, cheap and reliable corona test: a result within an hour
TNO, together with the GGD Amsterdam, will soon open a test street at the RAI for a Corona rapid test that is being developed by TNO and partners. This new test shortens the time between sample collection and actual detection of the COVID-19 virus to one hour with the same reliability as the PCR test. Being able to determine more quickly if a person is infected is vital in efficiently controlling the infection in the population. Samples from the GGD test line are assessed by the GGD by PCR and by TNO with the new test. The two are compared later.
A lot is involved when testing whether someone is infected with the corona virus. Various operations and steps are required to eventually arrive at detection. This takes a considerable amount of time and requires expertise, specific test kits and reagents. These are not always sufficiently available. With the help of a new detection method (LAMP) newly developed by TNO for COVID-19, the number of intermediate steps is simplified and reduced, as is the amount of reagents required. The price per test is therefore expected to be well below half of the current PCR test.
Molecular rapid test LAMP
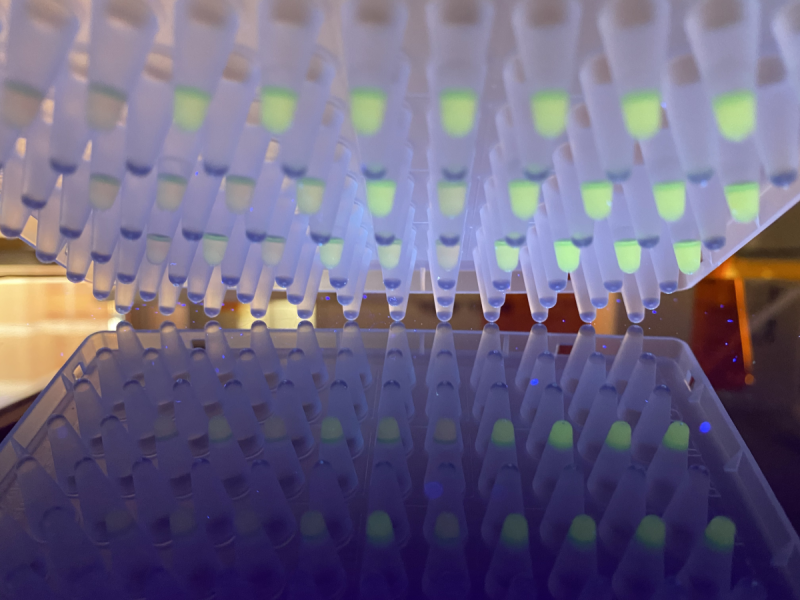
TNO is developing this method based on LAMP (Loop mediAted isothermal amPlification). LAMP is cheaper, less demanding with regard to the required laboratory infrastructure and gives faster results. For example, it is possible to work at a constant process temperature, in contrast to the current PCR method with a narrowly-defined sequence of temperatures and operations that is only possible in well-equipped labs.
LAMP therefore makes it possible to determine locally and well within one hour whether someone is infected. You can wait for the result, as it were, where it can now take 24-48 hours. LAMP can also be performed with materials exclusively produced in the Netherlands. The development is for the Ministry of VWS and in collaboration with the GGD Amsterdam, RIVM, DSM, LUMC and UMCG with funding from ZonMW.
Validation
The method, including the entire work process surrounding the test, is now being clinically validated in a fast track in collaboration with the GGD Amsterdam. After previous technical and clinical validation in laboratory conditions, the method is now being tested further under practical conditions and assessed for robustness and reliability in the trial test line at the RAI in Amsterdam.
After that, this easily scalable method can be introduced in the Netherlands. That would mean that sampling and direct assessment could be performed in many more places. It also relieves the pressure on the current test capacity.
National production
All materials and reagents can be produced in the Netherlands. National production is set up by DSM for the enzymes needed for the LAMP analysis. This makes the Netherlands independent of foreign suppliers and competition.
This project was started within the Brains4Corona initiative of TNO. The innovative strength of TNO employees is used in this to contribute to combating the effects of the corona virus. At the request of VWS, the project was funded as an urgent research project by ZonMW.